Background: Upfront autologous stem cell transplantation (ASCT) in multiple myeloma (MM) following induction therapy has been demonstrated to improve progression free survival (PFS) and overall survival (OS). Consideration of transplant eligibility involves assessment of age (typically <70 years), co-morbidities and frailty. In Australia and New Zealand, approximately 70% of all MM patients aged <70 years undergo upfront ASCT compared to approximately 6% aged 70-75 years (Bergin, MRDR Data). We aimed to review the patterns of transplantation in Australia and New Zealand in patients ≥70 years of age and examine survival outcomes and predictors of survival in this cohort.
Methods: We analysed 8786 MM patients who received ASCT in Australia and New Zealand between 2001 and 2019. 630 (7.2%) were ≥70 years of age. As there was missing data in the registry, additional data was obtained for 466 ≥70 years of age from 20 sites (performance status (PS), melphalan dose and creatinine clearance (CrCl)). These sites were selected on the basis of number of eligible patients in the registry. Kaplan-Meier analysis was performed to determine PFS and OS. Univariate and multi-variate analysis was performed using Cox proportional hazard model to determine predictors of OS.
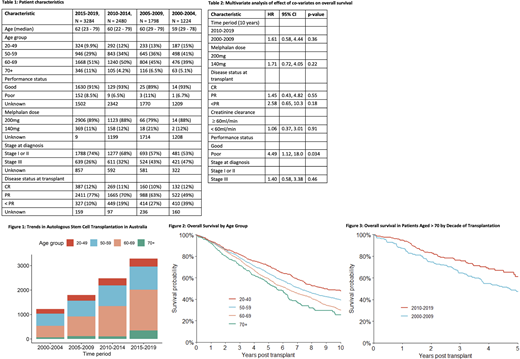
Results: The baseline patient and disease characteristics are presented in Table 1. The total number of ASCT procedures performed for MM has increased over the study period, and the proportion of ASCT patients ≥70 years has also increased from 5% in 2000-2004 to 11% in 2015-2019 (Figure 1). 33% of patients ≥70 years of age received reduced dose melphalan (140mg/m2 versus 200mg/m2) compared with 10% of patients < 70. Poor PS (ECOG > 1/Karnofsky Performance Score < 80) and CrCl did not significantly predict dose reduction of melphalan.
At a median follow-up of 3.8 years, median PFS was 3.3 years (95% CI 2.9-3.8) for those aged ≥70 and 3.4 years (95% CI 3.2-3.6) for those 60-69 (P =0.7). Median OS in those aged ≥70 was 5.6 years (95% CI 4.9-6.3) compared to 6.2 years in those 60-69 (5.8-6.6 years) (P = 0.01). There was no difference in median time to platelet and neutrophil engraftment in patients aged ≥ 70 compared to those < 70. There was no significant difference in transplant related mortality at day 100 in those ≥70 years (1.8%, 95% CI 1-3%) compared to those < 70 (1%, 95% CI 0.7-1.2%) (P = 0.07).
OS in all patients aged ≥ 70 (n = 630) was significantly better in patients transplanted between 2010-2019 (n = 451) compared to 2000-2009 (n = 179) (HR 1.62, 1.20-2.19, P = 0.002) (Figure 2) likely correlating with access to bortezomib based induction in 2011/2012 in Australia and New Zealand, and is reflected by an increased proportion of patients achieving a partial response (PR) or better at time of ASCT (Table 1). Increased access to novel agents in the relapsed/refractory MM patients as well as improvements in supportive care also may have contributed. On univariate analysis, other predictors of OS in older patients were poor PS (HR 2.44, 95% CI 1.23-4.81, P = 0.01), higher risk disease (Stage III using Durie-Salmon, ISS or R-ISS) (HR 1.42, 95% CI 1.01-2.00, P < 0.042) and failure to achieve a PR prior to ASCT (HR 1.71, 95% CI 1.01-2.87, P = 0.05). On univariate analysis, melphalan dose did not predict OS (HR 1.35, 95% CI 0.89-2.05, P = 0.2).
Multivariate analysis of determinants of OS was performed for the patients in whom we obtained the additional data. Because of missing data for both PS and stage, multivariate analysis incorporating all variables of interest (decade of transplant, melphalan dose, disease status at transplant, CrCl, PS and stage at diagnosis) could only be performed in a subset of patients (n = 163) (Table 2). In this cohort the only significant predictor of OS was poor PS (Table 2).
Conclusion: There is increasing utilisation of upfront ASCT in patients aged ≥ 70 in Australia and New Zealand. OS in this group of patients has significantly improved over the study period in keeping with access to bortezomib based induction and novel agents in the relapsed and refractory setting. In a highly selected group of patients ≥70 years of age, ASCT is feasible and associated with excellent PFS and OS. On multivariate analysis, PS was the only predictor of OS. The prospective use of established co-morbidity and frailty scores in assessing transplant eligibility in older patients warrants further evaluation.
Harrison:Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen-Cilag: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Consultancy, Honoraria; Haemalogix: Consultancy; AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; GSK: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; CRISPR Therapeutics: Consultancy, Honoraria; F. Hoffmann-La Roche: Consultancy, Honoraria; Janssen: Honoraria; Novartis: Consultancy, Honoraria, Patents & Royalties: wrt panobinostat; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Spencer:AbbVie, Amgen, Celgene, Haemalogix, Janssen, Sanofi, SecuraBio, Specialised Therapeutics Australia, Servier and Takeda: Honoraria; Celgene, Janssen and Takeda: Speakers Bureau; AbbVie, Celgene, Haemalogix, Janssen, Sanofi, SecuraBio, Specialised Therapeutics Australia, Servier and Takeda: Consultancy; Amgen, Celgene, Haemalogix, Janssen, Servier and Takeda: Research Funding. Mills:Celgene: Honoraria; Novartis: Honoraria, Other: Meeting sponsorship; AstraZeneca: Honoraria; Abbvie: Membership on an entity's Board of Directors or advisory committees. Hertzberg:Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Support of parent study and funding of editorial support; MSD: Membership on an entity's Board of Directors or advisory committees; Gilead: Membership on an entity's Board of Directors or advisory committees; BMS: Honoraria; Abbvie: Honoraria; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees. Sidiqi:Amgen: Honoraria; Janssen: Honoraria; Celgene: Honoraria, Other: Travel grant. Kalff:Celgene: Honoraria; Janssen: Honoraria; Amgen: Honoraria; CSL: Honoraria; Roche: Honoraria. Hamad:Novartis: Honoraria; Abbvie: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal